Some people claim hydrogen as being THE solution for the future energy source. Others are seeing methane as being the right answer to run through the energy transition, and even for further when produced on a renewable way. But let us deepen in the matter and analyse both, without a priori, purely along the energetic path.
Chemists are using moles, which are easy to work with in chemical reactions, gas transportation uses rather flow (volume by units of time) and pressure, but at the end of the day what tells is the transported energy (or more precisely its flow, thus the energetic power associated to this transport). So, yes, the following will require to spring from the one to the other, to enable a global comparison. Otherwise you keep focusing on one dimension, neglecting all the others so that no actual conclusion is possible. They will also be calculations and figures, so it will not be easy for everybody to stay on board, but I’ll try to make it as simple as possible.
Of course we must start by stating that the hydrogen and the methane considered here are produced only on renewable way (we will examine how). Hydrogen produced by steam reforming of methane or any other fossil fuels, is not considered. These transformations of fossil fuels to produce hydrogen, can only make sense when hydrogen is necessary as such, but definitively not from an energetic nor an environmental point of view.
Conversely, making methane from (renewable) hydrogen, could make sense from an energetic point of view if the consumed energy to achieve the transformation and pressurize the methane to pipe network operating level (60 bars in Belgium) is lower than (or equal to) the one necessary to pressurize hydrogen to an equivalent level (pressure at which the energy flow in the pipe is equivalent to the one in the methane network).
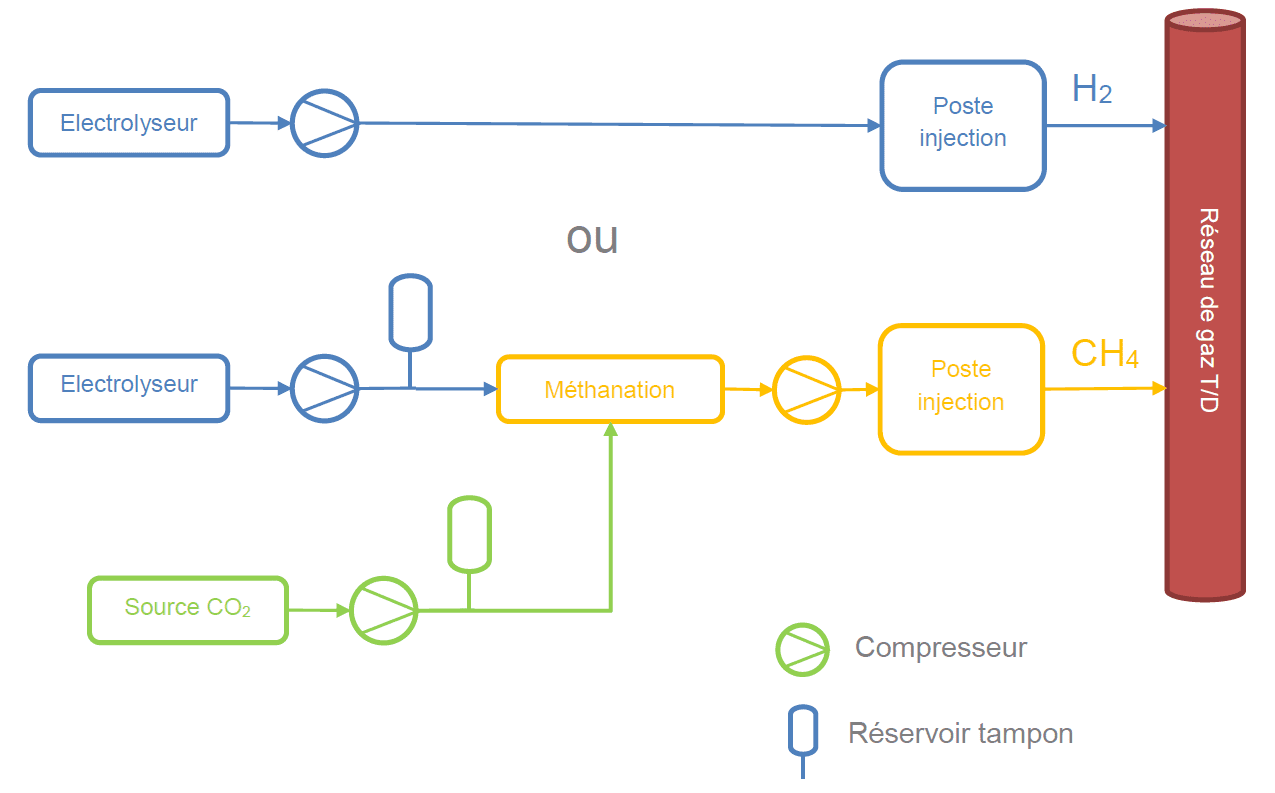
So let us assess and compare both paths:
- renewable hydrogen available at atmospheric pressure -> compression to necessary pressure (to be determined) -> distribution/delivery till end user
- renewable hydrogen available at atmospheric pressure + CO2 -> renewable methane at atmospheric pressure -> compression of methane to 60 bars
To analyse the 1st path, we must start with determining the pressure to which the (renewable) hydrogen must be compressed and then evaluate the energy necessary to achieve this work.
For the energetic content of a mole of hydrogen, as for methane, we will use the lower gross caloric value (GCV) of hydrogen (not the higher one, because this latest would suppose the total recovery of the latent heat of water vapour contained in the exhaust). The GCV of hydrogen is of 242 kJ/mole, the same figure for methane is of 803 kJ/mole, both are values at atmospheric pressure and 25°C, rounded at 0 decimal.
So we need roughly 3,318 moles of hydrogen to deliver the same energy as 1 mole of methane. According to the perfect gas law, this means that the pressure to which hydrogen must be compressed is 3,318 times the pressure at which methane is delivered, to reach the same level of energy flow (power thus). Considering a methane network pressure of 60 bar, hydrogen should be compressed to 200 bars.
The compression of gas is following a curve called a polytropic, taking into account the molecular specificities of the gas. Some people would argue that an isothermal compression would require less work than the adiabatic or polytropic one, forgetting it would require work to evacuate the produced heat in order to keep the temperature constant (isotherm). Lowest total work (compression + cooling) are obtained using multi-staged intercooled cycles. In that case, the real consumed energy to compress hydrogen to 200 bars is of 10 MJ/kg (20 kJ/mole). So the remaining useable energy is of 242 kJ/mole – 20 kJ/mole = 222 kJ/mole. We will come back later on the last step of this path, the distribution/delivery to the end user.
The 2nd path consists in 2 steps: a chemical reaction wherein CO2 is combined with H2 to give CH4 (methane), followed by the compression of the obtained methane to 60 bars. The step ‘distribution/delivery till end user’ is nowadays already achieved by the existing’ natural gas network. Some people will argue that CO2 must first be captured to be at disposal. That is right, but in a cycle where the (obtained methane) is burned (and thus releases the priorly captured CO2) and the exhaust stored to re-enter the cycle, after the 1st capture, no additional one is necessary. Further, industrial sized development have been made by specialized company enabling this capture of CO2, in a renewable way and requiring very few energy. Recent developments mentions the capture of CO2 from the atmosphere by specific weeds and release by rinsing them. So the capture of CO2 is not a step to take in consideration in our analysis.
The 1st step (methanation) in which methane is obtained from hydrogen and CO2, is exothermic, which means that it delivers energy instead of consuming energy. The freed up energy amounts 165 kJ by mole of methane generated (or by 4 moles of hydrogen consumed, since the chemical reaction is: 4 H2 + CO2 -> CH4 + 2 H2O). So for this step we have 4 x 242 kJ/mole entering in the process (the energy contained in 4 moles of hydrogen) and 803 kJ/mole (contained in 1 mole of methane) + 165 kJ/mole (freed-up), resulting in – 4 x 242 kJ/mole + 803 kJ/mole + 165 kJ/mole = 0 (checksum = 0, thus O.K.). So, if we can valorise the heat energy created by the reaction, this step will have a positive effect on the analysed path.
Of course, valorising heat energy is not obvious, transforming it in movement or electricity would undergo losses along the related thermodynamic cycle. So the only way to use it with (nearly) 100% yield, is to heat something. And we are lucky because, hydrolysis of water (the process that generates, upfront the hydrogen) is endothermic (requires heat input to be maintained) and thus reaches higher performance when the operating temperature rises, so we can use/valorize directly and on site (even in the same machine box, if designed so) the heat freed-up by the methanation. So, globally, this step has, indirectly, a positive energy balans (yield of electrolysis at 150°C is around 75% while it is of 50% at ambient temperature, at around 1100°C water cracks spontaneously in H2 and O2).
The 2nd step, the compression of methane to 60 bars, follows the same polytropic/adiabatic/isothermal … finally multi-staged intercooled process, but applied to another gas (i.e. methane) and to a lower pressure (60 bars instead of 200 bars for hydrogen). The compression of methane to 60 bars costs, for a multi-staged intercooled process, 0,7 MJ/kg (11 kJ/mole).
Thus, along the second path, the remaining useable energy is of, taking into account that the 1st step (methanation) has a null effect on the energetic level, 242 kJ/mole – (1/4) x 11 kJ/mole = 239 kJ/mole, some 7,6% above the one of the first path. But, if the freed-up heat is used to enhance the (upfront) electrolysis yield, let us say of 20% at industrial scale (50% in lab scale), we get then 287 kJ/mole.
Now, this is a sensitive but not huge difference (287 kJ/mole vs. 222 kJ/mole is 30% more) that may not occult other elements. The ones related to the distribution/delivery of the produced gas.
The thickness of a pipe/vessel in which a gas is transported or stored depends on the pressure of this gas in the pipe/vessel. The relationship between thickness and pressure is (really) linear, so for a pipe made of the same material and having the same diameter (e.g. the existing methane network). The required thickness of the pipe to transport gas at 200 bar is 3,318 times the one to transport a gas at 60 bar. This means that the existing methane network can’t be used to transport hydrogen (at the same energetic flow) and further that, if we would build a network to transport hydrogen, the pipes of this network would be much thicker (and thus heavier). This replacement would thus be very costly.
Further, hydrogen has also other annoying characteristics, like its diffusivity which will require or still thicker pipes or the use of other (special) material and special welding technics.
Finally, even at 200 bar, hydrogen is not yet at a pressure that is high enough to enable transportation use, which is driven by autonomy and small storage volume. For this type of usage, hydrogen must be compressed at 700 bar, which means a very high additional compression work would be required at each filling stations that would be fed with hydrogen (already) at 200 bar.
As a conclusion, it is obvious that, from an energetic and environmental point of view, (renewable) methane (obtained by methanation of hydrogen) is much better than (renewable) hydrogen, as energy vector, thus to transport/store renewable produced energy. For transportation on short- to mid- range (from daily commuting till 500-1000 km) electricity (in battery) is the best option, due to the poor yield of thermodynamic motor cycles (roughly 25%), as the too low one of the fuel cells (roughly 40%). Of course for industry, using hydrogen as feedstock, is (renewably produced) hydrogen better than methane.